Abstract
Background: Mantle cell lymphoma (MCL) is an uncommon B-cell lymphoma with heterogeneous clinical presentations and treatment response. Over the last two decades, the frontline therapy for MCL has evolved and we previously characterized this shift and the associated improvement in survival outcomes (Castellino, Blood Advances 2022). The evolved landscape of frontline therapy, as well as the emergence of new agents, may have impacted second-line (2L) treatment choice, response and survival. In this study we investigated the treatment patterns and survival outcomes for patients with R/R MCL treated with 2L therapy.
Methods: Consecutive patients with newly diagnosed MCL from September 2002 through June 2015 were offered enrollment in the Mayo Clinic/University of Iowa Molecular Epidemiology Resource (MER). All participants were prospectively followed for disease relapse/progression, subsequent treatments, and death. Patients who initiated 2L therapy for R/R MCL through 2021 were included in this analysis. Clinical characteristics, 1L, and 2L therapies were abstracted from MER and medical records. Treatment outcomes were calculated from the start of 2L therapy. Event-free survival (EFS) was defined as time from 2L initiation to progression, retreatment, or death due to any cause. Overall survival (OS) was defined as the time from 2L initiation to death due to any cause. A spline plot was generated to visualize the hazard ratio (HR) for EFS and OS over time from the initiation of 2L treatment. Three eras were defined based on the timing of 2L treatment: 2003-2009 (Era 1), 2010-2014 (Era 2), and 2015-2021 (Era 3). Eras were chosen to align with approval of novel therapies.
Results: Among a total of 343 MCL patients, with a median follow up of 7.6 years, 59 died without progression and 194 relapsed. Of those, 177 received 2L therapy and had complete treatment information available (N=58 in Era 1, N=71 in Era 2, N=48 in Era 3). At the time of 2L therapy, 127 (72%) patients had an age >60 years, 143 (81%) were male, 139 (79%) had stage III/IV disease, and simplified MIPI was low in 37 (34%), intermediate in 42 (39%), and high in 30 patients (28%; simplified MIPI missing in 68 patients). No statistical differences in age, sex, stage, or simplified MIPI were found among different eras.
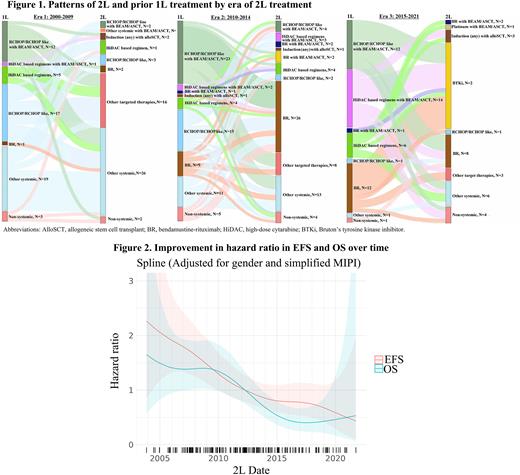
The patterns of 2L treatment by era, and previous 1L treatment, are shown in Figure 1. There were substantial heterogeneities in both 2L and 1L treatments across all eras. Notable changes in 2L treatment patterns were observed among different eras, likely related to drug availability and 1L treatment choices. Few to no patients received a Bruton's tyrosine kinase (BTK) inhibitor in 2L in Era 1 (N=0) and Era 2 (N=4; 6%), largely due to lack of availability, in contrast to Era 3 (N=21; 44%). The use of bendamustine-rituximab (BR) in 2L was minimal in Era 1 (N=2; 3%) and less in Era 3 (N=8; 17%) compared to Era 2 (N=26; 37%). High dose cytarabine use in 2L was higher in Era 1 (N=4; 7%) and Era 2 (N=4; 6%) compared to Era 3 (N=0). The percentage of patients who received autologous or allogeneic stem cell transplant appeared similar: Era 1 (N=5; 9%), Era 2 (N=10; 14%), and Era 3 (N=6; 13%).
The median follow-up from 2L therapy in Eras 1-3 was 13.3, 8.7, and 4.2 years, respectively. The overall response rate to 2L therapy was 57%, 79% and 83%, respectively (complete response rate 30%, 52% and 51%, respectively). The estimated 2-year EFS rate was 21% (95% CI: 13-34) in Era 1, 39% (95% CI: 29-52) in Era 2, and 58% (95% CI: 44-75) in Era 3. The estimated 5-year OS rate was 31% (95% CI: 21-46) in Era 1, 38% (95% CI: 28-52) in Era 2, and 69% (95% CI: 56-86) in Era 3. The spline plot depicts a trend in decreased hazard in EFS and OS over time (Figure 2). For example, compared to 2L start in April 2012 (median 2L start date, reference point), 2L start in January 2008 and January 2017 had a HR for EFS of 1.6 and 0.8 respectively, and a HR for OS of 1.4 and 0.4, respectively.
Conclusion: 2L therapy for MCL evolved with time and was likely affected by 1L treatment choice as well as the availability of treatment options at the time of need. The changes in 2L treatment correlated with improved EFS and OS, suggesting that treatment advances were associated with improved outcomes in patients with R/R MCL. Larger real-world studies are needed to further understand treatment patterns, changes with time, and the association with outcomes in R/R MCL, including 2L and subsequent lines of therapy.
Disclosures
Maurer:Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Research Funding; Roche/Genentech: Research Funding. Cerhan:BMS/Celgene: Research Funding; Genentech: Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; NanoString: Research Funding; Protagonist: Membership on an entity's Board of Directors or advisory committees. Ayyappan:Total CME: Speakers Bureau; TG therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle genetics: Membership on an entity's Board of Directors or advisory committees; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees; Fate therapeutics: Membership on an entity's Board of Directors or advisory committees; beigene: Membership on an entity's Board of Directors or advisory committees; Astrazeneca: Membership on an entity's Board of Directors or advisory committees; abbvie: Membership on an entity's Board of Directors or advisory committees. Ansell:SeaGen: Research Funding; Takeda: Research Funding; Bristol Myers Squibb: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Pfizer: Research Funding; ADC Therapeutics: Research Funding. Witzig:Curio Science: Honoraria; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Other: Clinical Trail Support; Kura Oncology: Other: Clinical Trail Support. Nowakowski:Bantam Pharmaceutical: Consultancy; Blueprint Medicines Corporation: Consultancy; Celgene Corporation/Bristol Myers Squibb: Consultancy, Research Funding; Curis, Inc.: Consultancy; Daiichi Sankyo Inc: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Genentech, Inc: Consultancy, Research Funding; Incyte: Consultancy; Karyopharm: Consultancy; Kite Pharma Inc.: Consultancy; Kymera Therapeutics: Consultancy; MorphoSys US Inc: Consultancy; NanoString: Research Funding; Ryvu Therapeutics: Consultancy; Selvita: Consultancy; TG Therapeutics: Consultancy; Zai Lab: Consultancy. Farooq:MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Honoraria; Caribou pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Checkmate Pharma: Research Funding. Wang:Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; InnoCare: Membership on an entity's Board of Directors or advisory committees, Research Funding; Loxo@Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Genentech: Research Funding; MorphoSys: Research Funding; Genmab: Research Funding; Eli Lilly and Company: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal